Technical Notes
COMET™ Assay Validation
The power of high-plex staining is to acquire the maximum of information in the same morphological context, from one single sample. The optimization and validation of an immuno-oncology multiplex panel with sequential immunofluorescence (seqIF) staining are challenging. Hands-on and manual adjustments are time-consuming and achieving reproducible results is tricky. The COMET™ System supports users in developing a first multiplex panel thanks to the integrated assisted optimization workflow (Figure 1). Once the optimization phase is completed, the validation phase establishes the system robustness as well as the precision of COMET™ assays, as shown in this technical note.
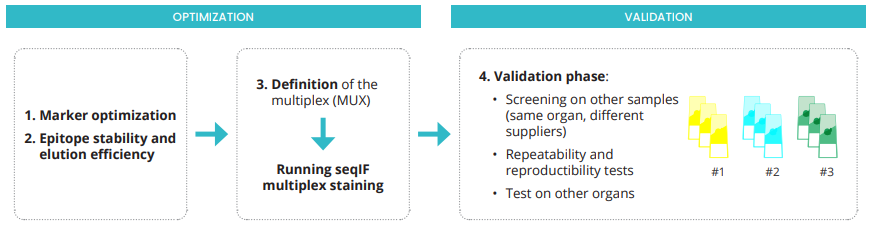
Figure 1. COMET™ workflow for optimization and validation of seqIF multiplex panels.
1. Protocol validation on other samples
The optimization phase is performed on tissue slides of the same organ and patient’s case. The first step of the validation phase consists of screening at least three different patient’s cases, ideally coming from three different tissue suppliers. A core immuno-oncology 10-plex panel, previously optimized on FFPE sections of human tonsil, is tested on three patient’s samples, provided by different tissue biobanks. Figure 2 shows that reproducible results are obtained on all different cases.
Related Articles
A user-friendly workflow from multiplexed images to data with HORIZON™
Posted on 09 Nov 2023
Read Post