Spatial Biology Education
The role of spatial biology in studying inflammation: A novel dimension
Posted on:
Inflammation is an essential response of our body’s immune system involving various cell types, effector molecules, and signaling pathways. However, chronic or uncontrolled inflammation can lead to various diseases, such as autoimmune disorders, sepsis, cardiovascular disorders, neurodegenerative disorders, and cancer. Thus, understanding the mechanisms and dynamics of inflammation is crucial for developing effective therapies and prevention strategies. Recently, spatial biology has emerged as a promising novel approach to study inflammation, as it enables researchers to visualize and analyze the spatial relationships between cells, tissues, and molecules within the context of the inflamed organ. By preserving the tissue integrity, researchers can identify immune cell subsets, investigate cellular interactions, and comprehend the localization of inflammatory mediators within the context of the tissue microenvironment. In this blog, we will explore the role of spatial biology in studying inflammation and highlight some of the latest findings and applications in this field.
Understanding cellular crosstalk and heterogeneity in inflammation
One of the remarkable features of inflammation is its spatial heterogeneity: different regions of the same tissue or organ can exhibit distinct cell composition and organization. Spatial biology allows researchers to dissect this spatial heterogeneity by characterizing and quantifying interactions of individual cells in distinct tissue locations. When inflammation is dysregulated, it can lead to devastating consequences, particularly in the context of chronic autoimmune diseases such as rheumatoid arthritis (RA). Montgomery et al.1 have identified and characterized two novel myeloid subpopulations in RA patients’ joints, among which a conserved group of tissue-resident monocytic-lineage cells (TR-MC). These TR-MCs have been found to possess unique cell surface markers, transcriptional programs, and thus, functions that set them apart from other immune cells. Even more intriguing, these cells exhibit rapid proliferation and reverse diapedesis in response to arthrogenic stimuli. Extravascular versus intravascular tissue location and deep phenotyping of this novel cell subset were assessed by high-throughput hyperplex experiments on the COMET™ platform. This groundbreaking research significantly enhances our understanding of RA and paves the way for developing innovative therapies to disrupt the inflammatory process, thus decreasing the burden of patients suffering from chronic joint inflammation.
Spatial dynamics and inflammation
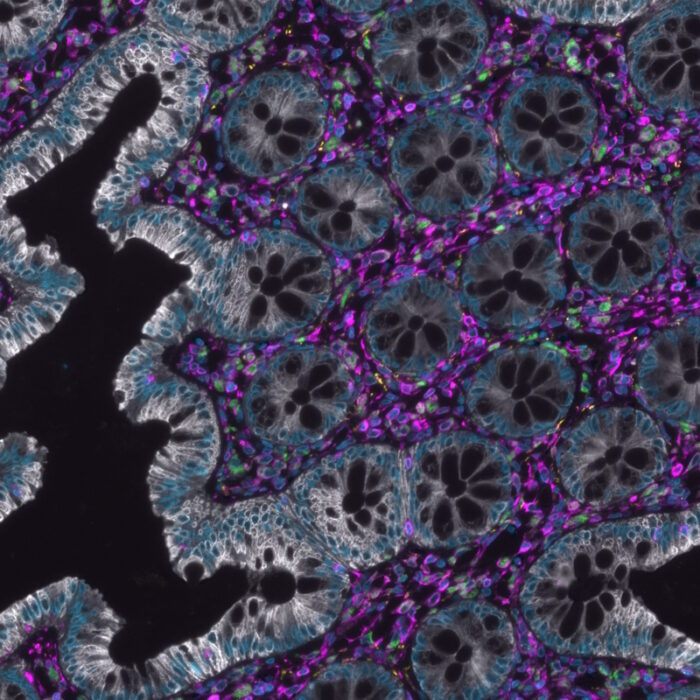
Inflammation is a dynamic process, and spatial biology can capture the dimensional changes of inflammation by monitoring the alterations in cell behavior, cytokine production, and tissue architecture over time and space. In the study by Ren et al.2, researchers found that vessel-associated macrophages (VAM) in the inflamed intestinal mucosa in mice produce a specific signaling molecule called TNF-alpha, that attracts polymorphonuclear neutrophils (PMN) to the site of inflammation. Using the hyperplex, high-throughput COMET™ platform, the authors confirmed the relevance of their findings in inflammatory bowel disease (IBD) patient biopsy specimens at the single-cell level (Figure 1). Due to the frequent correlation between the presence of PMN and tissue injury in IBD, as well as in other diseases settings, there is potential for therapeutic interventions by targeting specific macrophage populations and their disease promoting activities.2

Choosing the best technology for your research
Multiplex immuofluorescence represents an invaluable technique used in research to investigate the spatial interactions between different proteins. Technology breakthroughs in the field of spatial proteomics based on an innovative sequential immunofluorescence (seqIF™) approach on the COMET™ platform, offer fully automated, quick, and precise multiplex spatial analysis. By integrating COMET™ into a laboratory setting, researchers can benefit from the following features:
- Walk-away automation: Fully integrated staining and imaging workflow.
- Unmatched hyperplex throughput: 4 slides of 40-plex per run.
- Label-free primary antibodies: Use any antibody without the need for conjugation.
- Rapid and flexible panel development: Build a 20-plex panel with any markers from your library within 1 week.
Inflammation is a powerful and complex biological process and its dysregulation plays a role in numerous diseases. The emergence of spatial biology has revolutionized our ability to study inflammation, offering a new lens to observe the intricate cellular dynamics and spatial relationships within tissue context. Through spatial proteomics, researchers are gaining unprecedented insights into the inflammation process, potentially driving the development of innovative therapies for patients with high unmet needs.
References:
- Montgomery SA et al. Tissue-resident, extravascular Ly6c- monocytes are critical for inflammation in the synovium. Cell Rep. 2023. 42(5):112513. doi: 10.1016/j.celrep.2023.112513.
- Ren X et al. Macrophage-endothelial cell crosstalk orchestrates neutrophil recruitment in inflamed mucosa. J Clin Invest. 2023. e170733. doi: 10.1172/JCI170733.
Related Articles
The Spatial Biology Week™ 2022: the transforming power of spatial biology
Posted on 07 Nov 2022
Read Post