Spatial Biology Education
Lighten up your spatial biology journey: tips for selecting the perfect secondary antibody for immunofluorescence staining
Posted on:
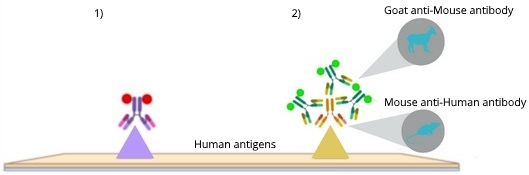
Immunofluorescence (IF) enables the detection and localization of a wide range of antigens in diverse cell and tissue types, and it is widely used in basic and translational research. Depending on the specific antibodies in use, we can distinguish direct IF (Figure 1.1), where the primary antibody is directly labeled with a fluorophore, and indirect IF (Figure 1.2), where the primary antibody is detected via a fluorophore-tagged secondary antibody1. The attached fluorophores have different excitation and emission spectra; therefore, they can be imaged using distinct light sources and filters.
Pros and cons of direct and indirect IF
Direct IF is recommended for highly abundant tissue antigens. On the other hand, indirect IF results in greater signal amplification, thus, is particularly beneficial for detecting low-expression proteins. Several secondary molecules will attach to each primary antibody thus amplifying the signal and increasing the assay’s sensitivity. In addition, secondary antibody conjugates are commercially available and relatively inexpensive, which avoids the need for laborious efforts to conjugate primary antibodies with fluorophores. Conjugation of the primary antibodies can also obstruct the antibody’s ability to recognize the antigen and lead to increased variability in results due to inconsistent labeling.
Despite the widespread use of IF, researchers frequently struggle to successfully detect a fluorescent signal from their target protein. In poor quality stainings, antibodies are often to blame. Various factors must be considered while selecting suitable antibodies.
How to select the best secondary antibodies for your experiments
We have previously discussed the importance of validating your primary antibodies (read more here). The correct choice of secondary antibodies is also essential to get reliable, high-quality data. We have collected these relevant tips to guide you in your selection of suitable secondary antibodies in order to achieve strong and specific fluorescent signal for your spatial imaging.
Consider secondary antibody host and target species:
- The primary antibody must target the species of the tissue sample to reliably detect the target epitope, while its host must be different from the tissue species in order to avoid getting background from the secondary antibody.
- The secondary antibody must target the host species of the primary antibody while being different from the species of the tissue. For example, a Goat anti-Mouse secondary antibody binds the Mouse anti-Human primary antibody which itself binds an antigen on a Human tissue sample (Figure 1.2). In the case of multiplex imaging (see below) on the same tissue using indirect IF, it is possible to detect two or more antigens at the same time. The antigens on the tissue sample are each targeted by primary antibodies that originated from different species and, subsequently, each primary antibody gets targeted by a secondary antibody, which bears a distinct fluorophore, that is specific to the primary antibody species. For example, on a Human tissue sample, a Goat anti-Mouse secondary antibody with fluorophore 1 binds the Mouse anti-Human primary antibody which is bound to antigen A and a Goat anti-Rabbit secondary antibody with fluorophore 2 binds the Rabbit anti-Human primary antibody which is bound to antigen B.
Select a secondary antibody that targets the class or subclass of the primary antibody
As an example, when working with primary polyclonal antibodies that are typically an immunoglobulin G (IgG), select a secondary antibody that is an anti-IgG. When a primary antibody is monoclonal immunoglobulin M (IgM) class, we recommend an anti-IgM secondary antibody.
Select affinity-purified antibodies
Affinity-purified antibodies are isolated from antiserum proteins using solid-phase affinity chromatography. Affinity-purified antibodies have an increased specificity and therefore offer a lower background signal. Using affinity-purified secondary antibodies also leads to less lot-to-lot variability.
Use cross-adsorbed antibodies
In addition to affinity purification, secondary antibodies can go through an additional purification process, called cross-adsorption, to avoid species cross-reactivity. During this process, serum proteins from different species are immobilized on an affinity chromatography column to remove undesirable reactivity from the secondary antibody pool. The use of cross-adsorbed antibodies is particularly beneficial when working with primary antibodies from various species in one experiment to minimize non-specific background and signal from a non-target primary antibody.
Select the correct fluorophore
The fluorophore conjugated to a secondary antibody is excited at a specific wavelength and emits light at a different wavelength. Amongst many commercially available conjugates, Alexa Fluor™ Plus secondary antibody conjugates are particularly interesting for spatial biology experiments as they have excellent brightness and photostability. One staining optimization possibility is to match a relatively bright fluorophore with a low expressed target.
Considerations in choosing secondary antibodies for multiplexed IF experiments
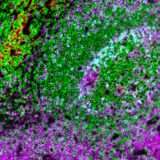
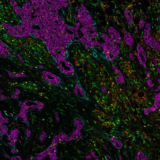
Using many fluorescent dyes at once to visualize numerous targets is known as multiplexing. Immunofluorescence for multiplex imaging (mIF) is used to generate spatial phenotypic signatures, which have proven successful in predicting therapeutic outcomes2. As mentionned earlier, the simplest way to multiplex is to use primary antibodies raised in different species in conjunction with species-specific secondary antibodies with various fluorescent dyes. When multiplexing, being able to discriminate between fluorophores is essential for obtaining accurate results. Make sure you examine the fluorophore’s excitation and emission curves to prevent fluorescence bleed-through. It is worth mentioning as well that cross-adsorbed antibodies are the most suitable for multiplexing as you can ensure that the signal reported by each secondary antibody is specific to each primary antibody.
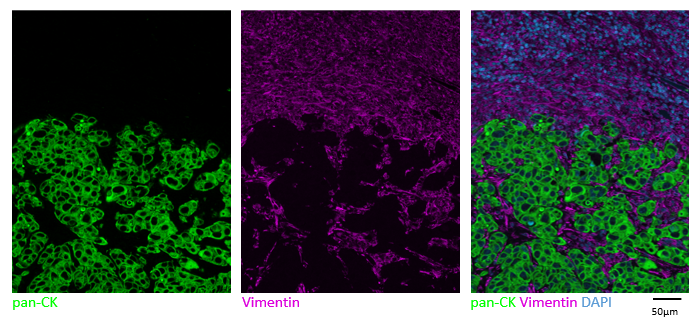
mIF is a powerful tool to accelerate your research, but it entails careful planning to yield reliable and reproducible data. Ensure you have all the right tools needed along the way. Obtain reliable results faster using the secondary antibody kits offered by Lunaphore, which can provide you with the following benefits:
- Purchase secondary antibodies in a convenient format and ready to use on your COMET™ platform.
- Obtain high-quality, reproducible results. The Lunaphore antibodies go through stringent quality control measures to provide lot-to-lot consistency, ensuring strong stainings with minimal background, and overall providing you with the reliability you need.
- Use a reliable solution providing high specificity. The Lunaphore pre-adsorbed antibodies ensure low species cross-reactivity.
- Benefit from Lunaphore’s markers and panels know-how, leveraging a wide range of pre-optimized protocols.
References:
- Kyuseok I. et al. An introduction to Performing Immunofluorescence Staining. Methods Mol Biol. 2019; 1897:299-311. doi: 10.1007/978-1-4939-8935-5_26.
- Massi D. et al. The density and spatial tissue distribution of CD8+ and CD163+ immune cells predict response and outcome in melanoma patients receiving MAPK inhibitors. J Immunother Cancer. 2019. 7(1):308. doi: 10.1186/s40425-019-0797-4.