Spatial Biology Education
The Spatial Biology Week™ 2022: the transforming power of spatial biology
Posted on:
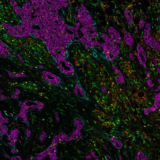
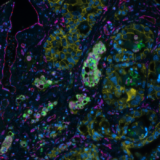
In recent times, biomarker discovery and analysis have become top priorities for the biopharma industry. Biomarkers allow researchers to gain a deeper understanding of the immunity and diseases’ cellular relationships within tissues. Identifying predictive biomarkers signatures can enable a more accurate stratification of patients in order to select the best treatment options, ultimately improving patient outcomes and quality of care. Spatial biology is a powerful tool to extract a large amount of biomarker contextual information from tissue samples by creating a cell-by-cell map of multiple dimensions and deciphering complex cell interactions in a spatial context. We can already see the value of predictive biomarkers in the spatial field; however, their identification and clinical applications can be difficult and time-consuming.
Spatial biology analysis, and more specifically, multiplex tissue analysis, can be performed by a number of techniques. One of them is multiplex immunofluorescence (mIF), which is becoming increasingly popular with the rise of cutting-edge spatial biology innovation that enables many labs to access advanced technology. Improved treatment approaches will result from making spatial technologies mainstream among research labs by using more reproducible and user-friendly solutions to ease their technology adoption process. The discussion about when and how such technologies can be utilized in clinical settings will soon be on the horizon.
During The Spatial Biology Week™, we sat down with the field’s top experts to discuss the benefits of spatial biology as well as the reproducibility and flexibility requirements for any research setting.
Reproducibility is crucial for accurate spatial discoveries
A common theme throughout the virtual event was the criticality of data reproducibility: the ability to obtain reliable information is needed in all stages of research, from basic research – when screening for new biomarkers – to translational and clinical research – when analyzing large patient cohorts. Reproducibility eliminates variability and allows researchers to perform controlled experiments and obtain actionable data.
For example, Dr. Michael Surace, Director at AstraZeneca, said, “Reproducibility is one of those dark horse metrics. If you don’t have reproducibility, and you aren’t measuring it, you may indeed have a real challenge in the longitudinal comparison between samples, but you may be unaware of it.”
At the end of the day, if data cannot be reproduced, the research study will be stuck at square one. For this reason, a spatial analysis platform that offers high reproducibility is vital to delivering robust data for the demanding requirements of translational and clinical research, especially when it aims to be used in the routine diagnostics environment.
The spatial exploration of biomarkers requires flexibility
Throughout The Spatial Biology Week™ 2022, the expert speakers mentioned versatility and flexibility as key requirements for spatial biology platforms. For example, having the freedom to choose any standard antibody without the need to perform any upstream conjugations or barcoding, brings a great degree of flexibility and simplifies assay development. Another requirement for a flexible platform is to include detailed customization possibilities when creating protocols in order to run different types of assays, develop and modify biomarker panels adapted to specific research questions in short amounts of time, as well as provide standard file format outputs so that users can utilize any preferred software tools to analyze the data. All of these aspects are essential to facilitate the adoption and integration of these platforms into a lab’s workflow. Dr. Priyank Patel, Senior Scientist at Boehringer Ingelheim, said on Day 2 of The Spatial Biology Week™, “We really like that COMET™ is an open platform; it gives us a lot of possibilities to optimize antibodies based on our criteria and develop custom panels. It gives us flexibility in designing these panels too.”