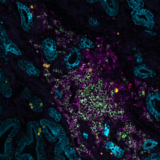
Poster
Deepening understanding of tumor budding in colorectal cancer for effective immunotherapy
Posted on:
Colorectal cancer (CRC) is one of the most prevalent cancers and causes of cancer-related deaths. The histologic type of cancer, the disease’s stage, the tumor-node-metastasis (TNM) staging system, and the status of the circumferential resection margin (CRM) are typically used to determine the best course of treatment. However, they only give a partial picture because many patients with projected early-stage disease have systemic and lymph node micrometastases, which may lead to disease recurrence1. These findings highlight the shortcomings of current techniques for accurately stratifying patients with CRC and the urgent need to find biomarkers that can provide clinicians with insights into prognosis and therapy choices.
What is tumor budding?
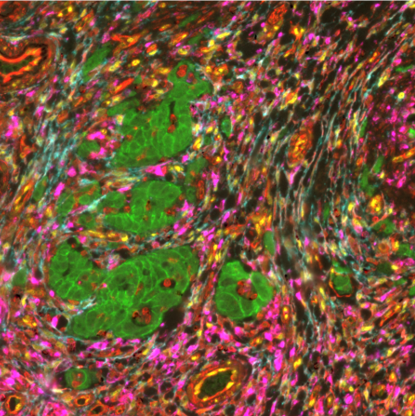
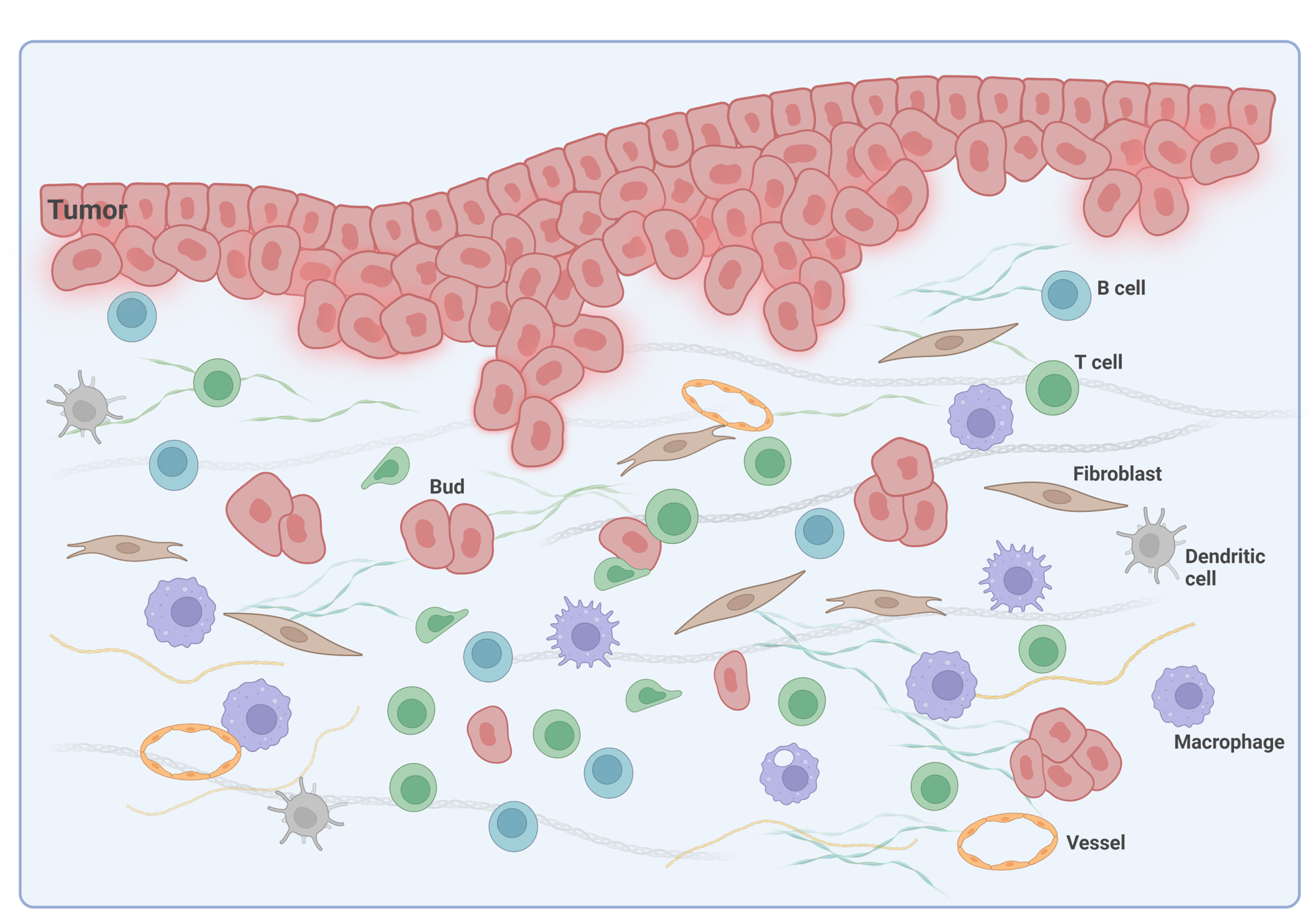
Tumor budding, defined as the existence of a single tumor cell or clusters of up to 4 tumor cells, is an emerging prognostic biomarker in CRC2,3. It has been linked to epithelial-mesenchymal transition (EMT) in the tumor microenvironment (TME) of CRC (Figure 1) and poor disease-specific survival rate4.
In pT1 colon cancer patients, tumor budding categories are utilized to determine if to conduct radical surgery after endoscopic resection. In stage II colon cancer, tumor budding is an indicator considered for the guidance of adjuvant treatment decisions5, which is a form of treatment used in conjunction with primary therapy in order to increase the effectiveness of both. In stage II CRC, it was also demonstrated that the spatial interaction between T lymphocytes and tumor budding holds significance as individuals with more lymphocytes around tumor buds show a better prognosis6. The recent post-hoc analysis of the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) France phase III study demonstrated that tumor budding also offers additional and clinically relevant prognostic information in patients with stage III colon cancer5.
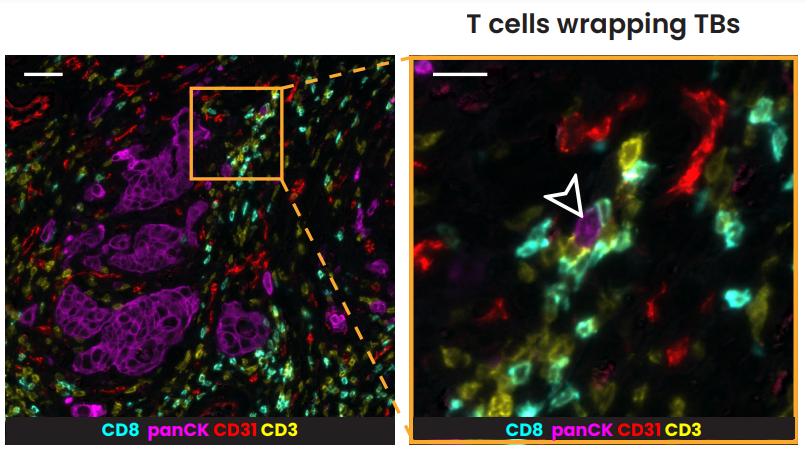
The evaluation of budding via multiplex immunofluorescence
Multiplex immunofluorescence (mIF), a new cutting-edge method, makes it possible to detect numerous proteins on the same tissue giving insights into their spatial organization. In the research conducted in collaboration with the University of Bern, sequential immunofluorescence (seqIF™) was used to spatially characterize tumor budding and TME in colorectal cancer. SeqIF™ consists of subsequent cycles of mIF staining, imaging, and elution, where two biomarkers are detected simultaneously in every cycle. The panel of 26 biomarkers was optimized to characterize the tumor and the surrounding stroma (Figure 2). The primary study findings indicated the loss of EpCAM and E-Cadherin in a group of tumor buds, verifying decreased epithelial phenotype (Figure3).
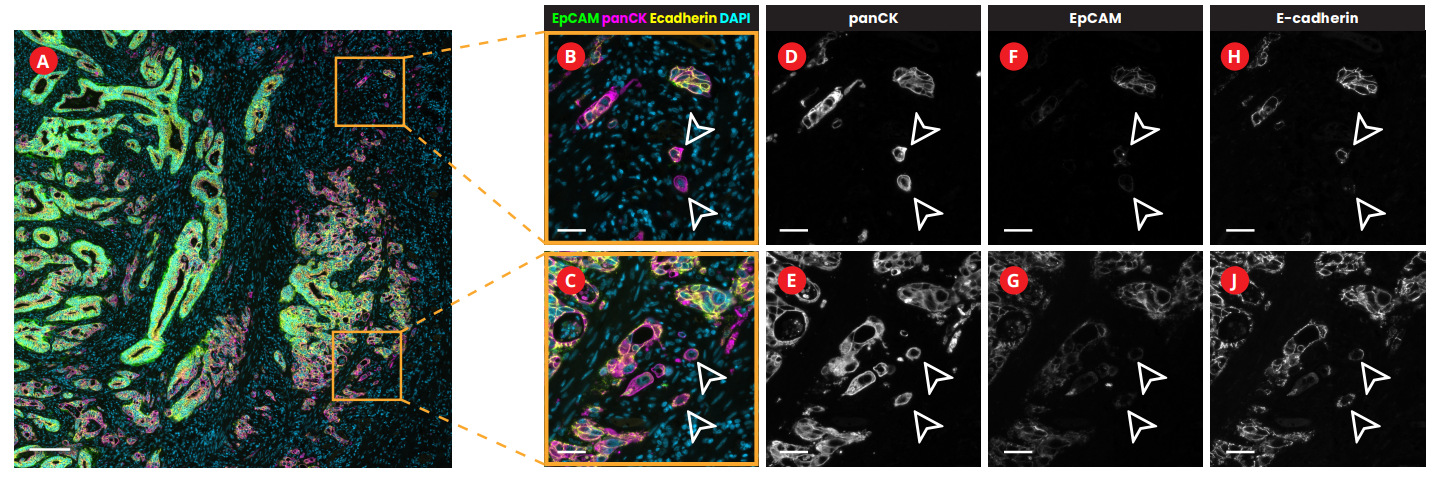
Growing data suggest that tumor buddings represent a phenotype-enhancing tumor invasion, reflecting the biological aggressiveness of cancer cells. In CRC, tumor budding is well recognized as a prognostic factor; however, there is still uncertainty on how tumor budding cells interact with stromal cells. Deepening our spatial biology understanding using mIF tools is essential for the development of immunotherapy targeting the destruction of tumor buds.
References:
- Chand M. et al. Novel biomarkers for patient stratification in colorectal cancer: A review of definitions, emerging concepts, and data. World J Gastrointest Oncol. 2018; 10(7):145-158.doi: 10.4251/wjgo.v10.i7.145
- Lugli A. et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016; Modern Pathology. 2017; 30(9):1299-1311. doi: 10.1038/modpathol.2017.46.
- Basile D. et al. Tumor budding is an independent prognostic factor in stage III colon cancer patients: a post-hoc analysis of the IDEA-France phase III trial (PRODIGE-GERCOR). Ann Oncol. 2022; 33(6):628-637. doi: 10.1016/j.annonc.2022.03.002. Epub 2022 Mar 16.
- Zlobec I. and Lugli A. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget. 2010; 1(7):651-61.doi: 10.18632/oncotarget.199.
- Basile D. et al. Tumor budding is an independent prognostic factor in stage III colon cancer patients: a post-hoc analysis of the IDEA-France phase III trial (PRODIGE-GERCOR). Ann Oncol. 2022; 33(6):628-637.doi: 10.1016/j.annonc.2022.03.002.
- Nearchou I. et al. Automated Analysis of Lymphocytic Infiltration, Tumor Budding, and Their Spatial Relationship Improves Prognostic Accuracy in Colorectal Cancer. Cancer Immunol Res. 2019; 7(4):609-620. doi: 10.1158/2326-6066.