Spatial Biology Education
Unlocking the secrets of spatial biology: a journey towards understanding and improving immunotherapy outcomes
Posted on:
Our bodies comprise billions of complex cells and tissues that keep us alive and functioning. Every single cell in our body is connected in some way to an elaborate network that helps us regulate our physiology. That is why studying the spatial environment of organisms remains one of the most important fields of research today. However, there are still several obstacles to the widespread use of spatial biology techniques. In particular, the level of complexity and the accessibility of learning opportunities can be in the way of gaining a better understanding of when and how to use those tools.
The use of biomarker screening can support improvements in the outcomes of biopharma immuno-oncology studies
Patient identification for optimal treatment has become possible due to the surge of predictive biomarker signatures, which can serve as powerful tools to determine the likelihood of response, or lack of response, to a particular therapy. Biomarker signatures enable the stratification of patient populations, improving therapy response rates in clinical trials, and therefore, they hold considerable potential to optimize both biopharma investment as well as patient outcomes.
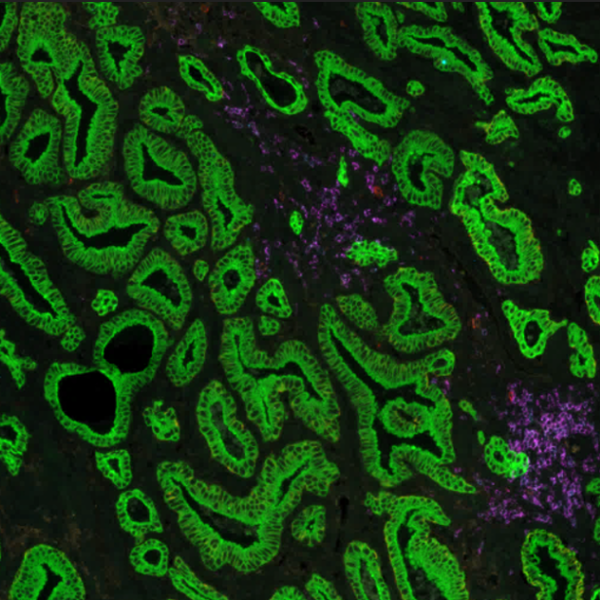
Identifying biomarkers with good predictive value requires phenotyping tissue samples of different patient populations and disease states. Gaining insights into the spatial immune environment of tumors is necessary for a thorough understanding of molecular changes that govern disease progression and impact immunotherapy response rates across different populations. Biopharma researchers require versatile tools that offer high-plex analysis capabilities, high-throughput automation, and high reproducibility to fulfill their translational needs. The use of biomarkers in research and clinical studies has grown exponentially in recent years (Figure 1). Since the human genome was sequenced, the use of biomarkers as inclusion or exclusion criteria for enrolling patients in clinical research has substantially expanded1. Some studies have documented the significant impact that biomarker selection can have on regulatory approval rates, demonstrating that a likelihood of a successful transition from Phase I up to Approval is 26% when performed with biomarker selection versus 8.4% without. It is clear that biomarker selection provides a critical tool for improving the efficacy and speed of clinical research.