Poster
The future of biomarkers: going “spatial”
Posted on:
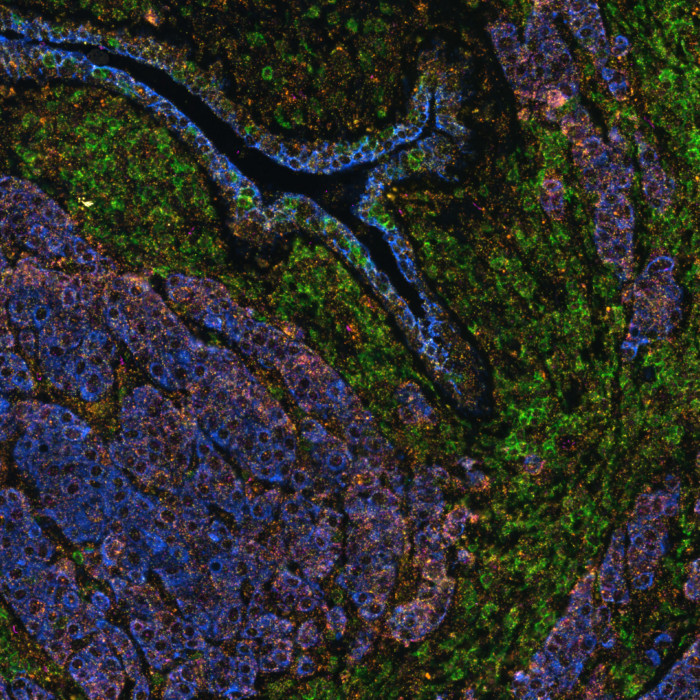
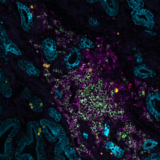
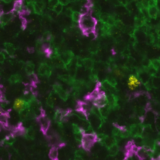
Organs are intricate ecosystems composed of hundreds of billions of diverse cells, each playing a crucial role in development, physiology, and disease progression. Understanding how these cells interact and organize within tissues is fundamental to decoding the complexities of biological systems. Spatial multiomics emerges as a revolutionary tool in biological research, allowing for the visualization and analysis of these intricate cellular relationships. This transformative approach not only provides a comprehensive view of living systems but also offers invaluable insights into disease mechanisms, biomarker discovery, and therapeutic innovation.
Spatial multiomics: visualizing molecular landscapes
Spatial multiomics represents a cutting-edge paradigm in biological research, seamlessly integrating multiple omics techniques. This innovative methodology combines data from various molecular layers and empowers researchers to analyze the molecular composition of biological samples while preserving their spatial context within tissues. While well-established proteomics and transcriptomics play pivotal roles in spatial multiomics, the continuous emergence of novel technologies such as epigenomics, metabolomics, and lipidomics underscores ongoing advancements in the field.
The significance of spatial biomarkers in biomedical research
Spatial biomarkers are biomarkers that exhibit specific spatial patterns within biological samples. Unlike traditional biomarkers, which are often measured in bulk samples or using tissue dissociated samples, spatial biomarkers provide information about the localization or spatial organization of molecules within their native context. These biomarkers and their spatial distribution may correlate with various physiological or pathological conditions and be associated with the patient’s potential for therapy response.
Spatial multiomics plays a pivotal role in biomarker discovery, offering several key advantages:
Related Articles
Cytokines, spatial activation and tumor infiltration: the future of immunotherapy
Posted on 13 Dec 2023
Read Post