Technical Note
Cancer research: why plex level matters in the development of cancer diagnostics and therapies
Posted on:
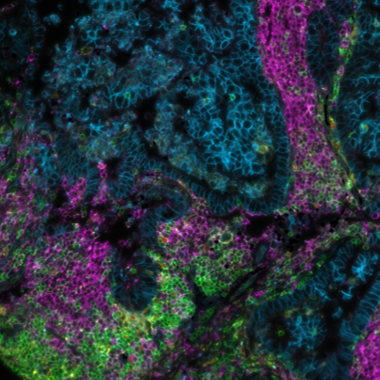
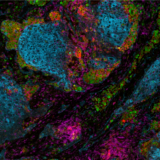
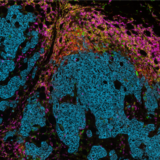
A crucial aspect of cancer treatment is understanding how the immune system interacts with tumor cells. Such knowledge is essential for the design of immunotherapies that use the human immune system to combat diseases. However, the clinical efficacy of immunotherapies is limited to a subgroup of patients. New strategies that can increase immunotherapy’s effectiveness are urgently needed. Identifying specific and predictive biomarkers for patients’ stratification and improving knowledge of the tumor microenvironment (TME) is critical. Multiplex immunofluorescence (mIF) approaches platforms have proven to be powerful tools to study the tumor immune context. They can concurrently identify multiple epitopes and obtain information about spatial relationships between various cell types in distinct locations. They provide high-quality, robust assays for analyzing cancer specimens at various stages of therapy and offer distinctive biological information that is frequently impossible to obtain using other techniques. Using spatial profiling for critical tumor-immune pathways can improve the classification of cancer patients for immunotherapy1 and increase their efficacy in clinical settings.
Multiplex technologies accelerate predictive biomarker development.
A companion diagnostic is a test for a predictive biomarker that stratifies patients into responders and non-responders for a given therapeutic. Companion diagnostic tests are currently used in clinical practice to identify and classify tumors using immunohistochemistry (IHC); however, IHC application is hindered by high inter-observer variability and the restriction to a maximum of one visualized marker per tissue section. High-throughput multiplex immunofluorescence could fulfill this gap thanks to the generation of high-quality, reproducible data. It offers the chance to obtain diagnostic and prognostic data that exceeds the highest standards.
In addition, multiplexing techniques are indispensable in the biomarker discovery phase, where spatially resolving many proteins is incredibly desirable. Therefore, researchers are pursuing novel biomarker signatures that capture the spatial context using multiplexed assays. For example, Yeong et al. 2. demonstrated the concordance between IHC and multiplex immunohistochemistry/immunofluorescence (mIHC/IF) by combining multiple different programmed death-ligand 1 (PD-L1) antibody clones in triple-negative breast cancer (TNBC) applied to a single tissue section. Adopting mIHC/IF in clinical settings could significantly improve the treatment of therapeutically challenging tumors. In another study, Feng et al.3 showed that mIHC/IF might be used for multiparametric immune profiling, which may benefit clinical trial stratification of patients with oral squamous cell carcinoma (OSCC). The authors demonstrated that high CD8+ T cell counts near the invasive margin significantly correlate with a better survival rate. Notably, the location of suppressive factors, in addition to their composition, is essential. Cells that are PD-L1+ or FOXP3+ and close to CD8+ T-cells have lower overall survival rates. Such results provide new opportunities to discover novel biomarkers and stratify patients.
COMET™ as a solution for reproducible hyperplex stainings
Related Articles
Reproducibility and tissue integrity: spatial biology without compromises
Posted on 08 Apr 2022
Read PostOncology: expanding the research frontier with revolutionary spatial proteomics
Posted on 29 Jun 2022
Read Post