Poster
Exploring spatial insights between tumor and stroma with multiplex immunofluorescence
Posted on:
For a long time, the focus of the development of targeted tumor therapeutics was on the modulation of changing processes in the tumor cell. However, the tumor microenvironment (TME) (Figure 1) and its stroma are becoming more and more interesting for therapy options. The stroma comprises various components, including the extracellular matrix and specialized connective-tissue cells such as fibroblasts and mesenchymal stromal cells1. It has been shown that the stroma plays critical roles in carcinogenesis, metastasis, and resistance to therapy. Here are some of the ways how the tumor stroma influences cancer therapy:
- Drug delivery: the tumor stroma can affect drug delivery to cancer cells by creating a barrier that limits the penetration of drugs into the tumor2, reducing their effectiveness. Understanding the stromal components and how they affect drug delivery can help researchers design better therapeutic strategies.
- Resistance to therapy: tumor stroma can contribute to resistance to therapy. For example, cancer-associated fibroblasts (CAFs) within the stroma can secrete cytokines, chemokines, growth factors, exosomes, and desmoplastic reactions, which protect cancer cells against drug-induced apoptosis 3. Targeting these factors or the CAFs themselves may increase the effectiveness of cancer therapies4.
- Immunotherapy: The tumor stroma can also impact the immune response to cancer. Immune cells within the stroma can promote or inhibit the immune response to the tumor5. Understanding the role of the stroma in immune regulation can help researchers design more effective immunotherapies.
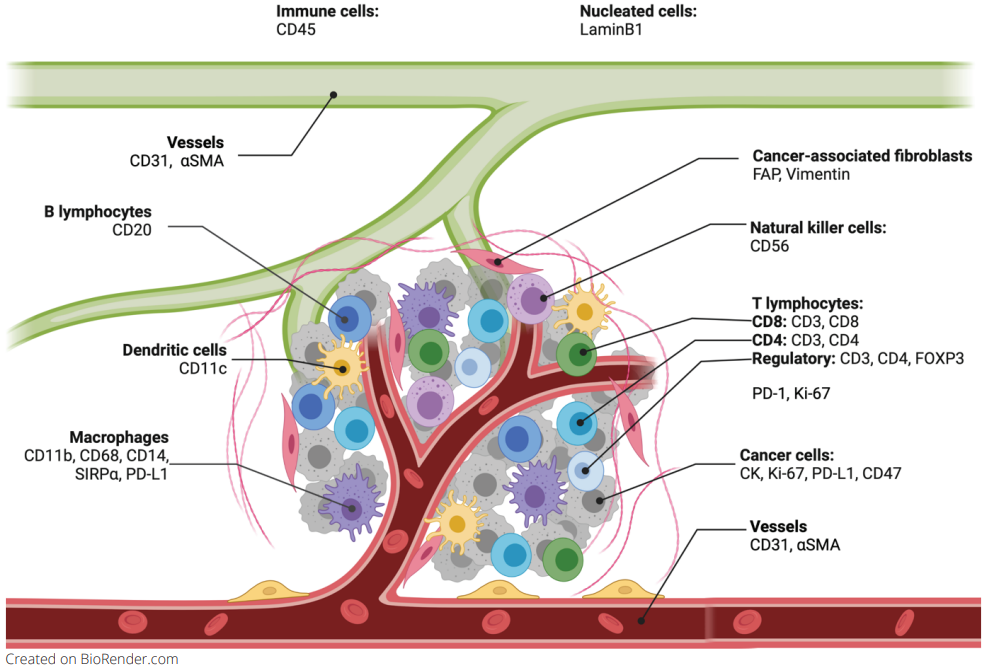
Spatial biology can give enormous insights into the role of the tumor stroma, the relationship between its components, the interaction with tumor and immune cells and therefore support the finding of new therapeutic approaches.
Reliable antibodies for multiplex immunofluorescence to analyze tumor-stroma interactions
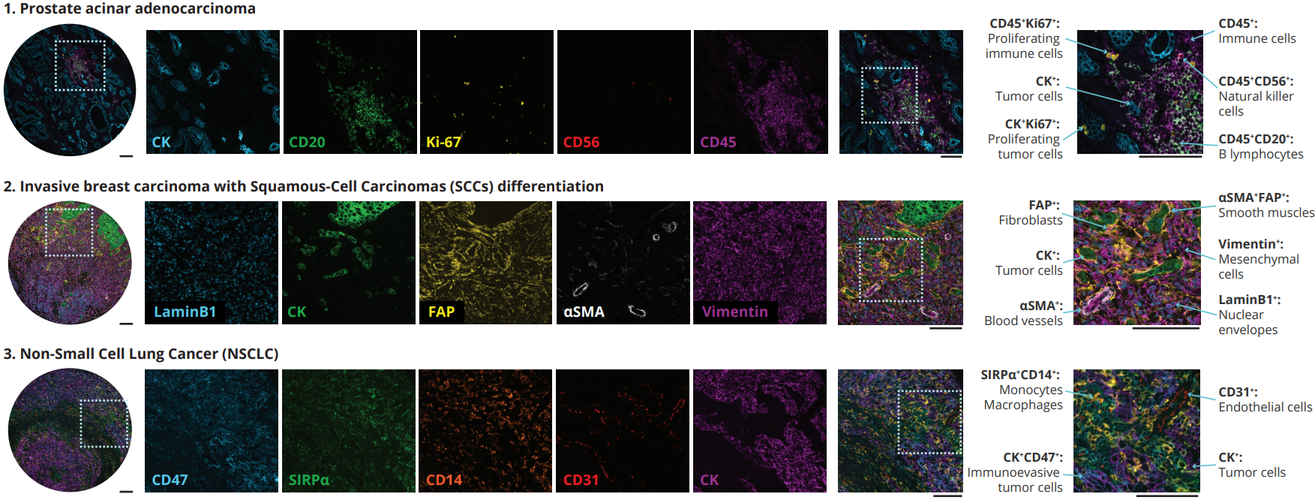
In a recent study exhibited at the AACR 2023 annual meeting, Lunaphore identified and characterized a list of markers to characterize non-tumoral immune cells, fibroblasts, and endothelial cells in the TME, in a single tissue slide via sequential immunofluorescence (seqIF™) on the fully automated, high-throughput, hyperplex platform COMET™. SeqIF™ consists of consecutive cycles of staining, imaging, and gentle antibody elution in an automated manner without the need for user intervention. Subsequently, another staining round can be conducted to visualize a new set of antigens. Two antibodies from different species are used in each cycle. Then, the acquired images can be stacked to create overlays to build the spatial relationship map. Post-processing of generated images was done with HORIZON™ image analysis software.
This panel was created using SPYRE™ Immuno-Oncology Core Panel and additional antibodies from abcam. The new SPYRE™ Antibody Panel kits have been added to the Lunaphore offering to help kick-start assay development with verified biomarkers. Lunaphore partnered with a leading antibody supplier, abcam, ensuring the best tools for spatial biology research. The agreement enables researchers to use abcam’s primary antibodies without conjugation directly on the COMET™ platform, with unparalleled scalability and reproducibility. By using validated primary antibodies together with the capabilities of COMET™, researchers can accelerate assay development processes and achieve reliable results.
The comprehensive panel of 22 markers allowed the assessment of multiple biomarkers on a single tissue slide on multiorgan TMAs. The full list of the markers via poster can be downloaded here. Using the seqIF™ protocol on COMET™ facilitated the study of colocalization and co-expression of markers otherwise not compatible with the simultaneous examination in the traditional immunofluorescence approach. The new hyperplex data unveiled substantial differences within the stroma between various types of tumors, emphasizing the significant heterogeneity of tissue composition and its intricate architecture (Figure 2). This panel enables the study of stroma, tumor, and immune cells and their reciprocal interactions.