Infographic
The spatial edge in meningioma research
Posted on:
Meningiomas are the most common primary intracranial tumors1. High grade meningiomas have long posed a challenge in oncology due to limited treatment options. While surgical resection and radiotherapy have been the primary approaches, systemic therapies have proven ineffective, leaving patients and clinicians in need of innovative solutions. However, recent breakthroughs in spatial biology are shedding new light on meningiomas, providing critical insights that could lead to more targeted and successful treatments.
The spatial edge
Meningiomas manifest in a multitude of forms. From benign to aggressive tumors, their heterogeneity at cellular and molecular levels has been challenging to understand2. Spatial biology, at its core, emphasizes the importance of location and interaction. It deciphers the spatial distribution of cells, tissues, and their molecular constituents. By embedding the principles of spatial biology in meningioma research, researchers aim not only to observe cells but also to understand their spatial organization, potential interactions, and microenvironment influences.
Spatial biology’s contribution to meningioma research
Characterizing tumor heterogeneity
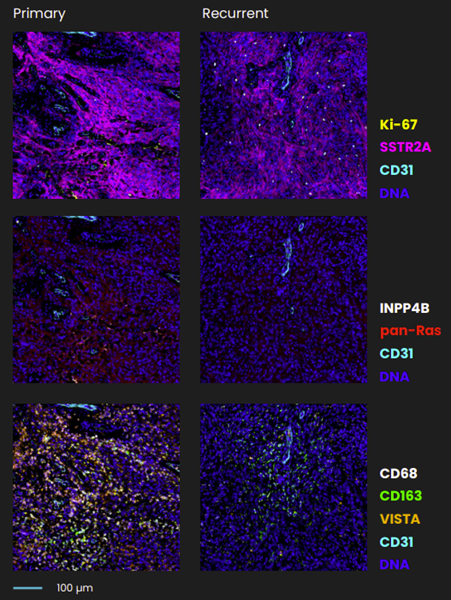
Varied pathogenic profiles of meningiomas, even within a single tumor, highlight a profound cellular heterogeneity. Spatial biology techniques, such as multiplex immunofluorescence (mIF), enable researchers to identify and characterize different cell populations within a tissue section. By preserving the architecture and localization of cells within their native microenvironment, mIF provides a multidimensional perspective of tissue organization and cellular interactions. Lucas et al. combined spatial transcriptomics, single-cell RNA-sequencing, and spatial proteomics3, including sequential immunofluorescence (seqIF™)4 on COMET™. The study used this multimodal approach to characterize the intratumor heterogeneity in low- versus high-grade meningioma and primary versus recurrent tumors using matched patient biopsies3 (Figure 1).
Identifying meningioma molecular drivers
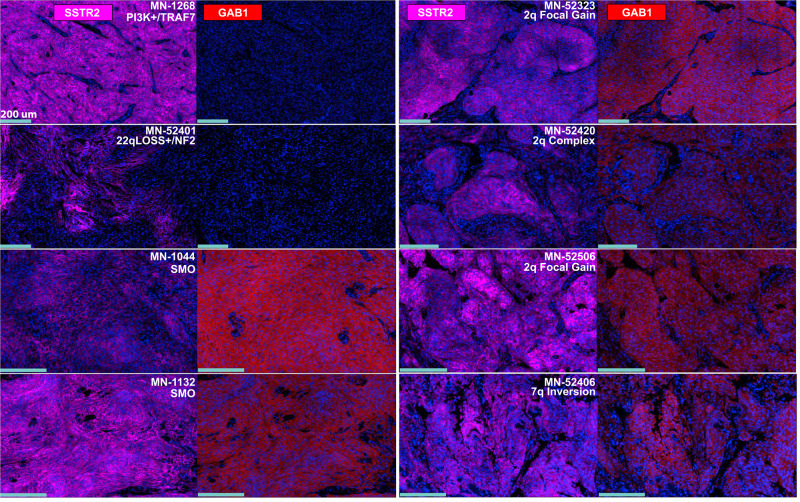
By mapping the spatial distribution of specific molecules within a meningioma, researchers can gain insights into the tumor’s composition. For example, identifying areas with high levels of proliferation markers can pinpoint regions of active tumor growth, which can guide treatment decisions. In addition, spatial biology reveals consequences of disturbance in known pathways that can be dysregulated in diseased samples. A recent study by Youngblood et al. found that 35.6% of the meningiomas that did not exhibit known molecular drivers, presented unique structural changes, specifically in chromosomes 2q35 and 7q36.3 (Figure 1). A closer look showed that these alterations lead to an abnormal expression of Hedgehog ligands – IHH and SHH, kickstarting Hedgehog (Hh) signaling. Moreover, certain repeated sequence changes near IHH result in entirely new interactions between powerful gene regulators and the IHH locus5.
Exploring new therapeutic targets
Spatial biology helps understand the current state of a meningioma and reveals potential therapeutic targets. Examining the spatial relationships between different molecules within a biological tissue is a fundamental aspect of spatial biology that can indicate critical insights into disease mechanisms and identify potential targets for therapy. The groundbreaking research paper by Choudhury et al. identifies NOTCH3 as a crucial driver of meningioma tumorigenesis and resistance to radiotherapy6.
The road ahead
The field of meningioma research is undergoing significant transformation thanks to novel insights from spatial biology. As our exploration into these tumors becomes increasingly detailed, we gain a clearer understanding of their behavior, cellular dynamics, and molecular patterns. Such discoveries present the potential for more precise, effective treatments and bring hope to address the longstanding challenges associated with these complex tumors.
Unlock a visual journey!
Would you like a concise introduction to the impact of spatial biology on neurosciences? Check out our latest infographic for an easy-to-understand summary of this complex field. It’s perfect for sharing with colleagues or using as a reference in your studies. Click below to explore the world of neurosciences from the perspective of spatial biology
References:
-
- Goldbrunner R. et al. EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. 2021. doi: 10.1093/neuonc/noab150.
- Magill S. et al. Multiplatform genomic profiling and magnetic resonance imaging identify mechanisms underlying intratumor heterogeneity in meningioma. Nat Commun. 11(1):4803. doi: 10.1038/s41467-020-18582-7.
- Lucas et al. Spatial genomic, biochemical, and cellular mechanisms drive meningioma heterogeneity and evolution. Res Sq. 2023. doi: 10.21203/rs.3.rs-2921804/v1.
- Rivest F. et al. Fully automated sequential immunofluorescence (seqIF) for hyperplex spatial proteomics. Sci Rep. 13(1):16994. doi: 10.1038/s41598-023-43435-w.
- Youngbloot et al. Super-enhancer hijacking drives ectopic expression of hedgehog pathway ligands in meningiomas. Nat Commun. 2023. doi: 10.1038/s41467-023-41926-y.
- Choudhury et al. NOTCH3 drives meningioma tumorigenesis and resistance to radiotherapy. bioRxiv preprint. https://doi.org/10.1101/2023.07.10.548456.