Spatial Biology Education
Assay development: how to overcome the challenges of TSA-based mIHC assays
Posted on:
What value do multiplex assays bring to cancer research?
Understanding how the immune system interacts with tumor cells is crucial when developing immunotherapies for cancer treatment. This class of therapies uses the human immune system to combat diseases. However, the clinical efficacy of specific immunotherapies is limited to a subgroup of patients. New strategies to increase the effectiveness of immunotherapies and improve the identification of patients that will benefit from a particular therapy are urgently needed. Research in this field hinges on identifying specific and predictive biomarkers for patient stratification and an improved understanding of the tumor microenvironment (TME).
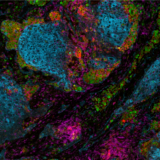
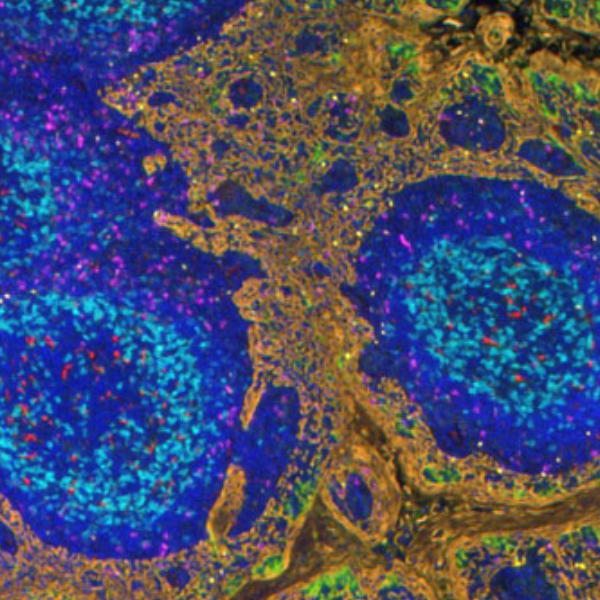
Multiplex immunohistochemistry (mIHC) is a powerful and proven tool to study the tumor immune context. With mIHC, researchers can concurrently identify multiple epitopes and obtain information about spatial relationships between various cell types in distinct locations. Using spatial profiling for critical tumor-immune pathways can improve the classification of cancer patients for immunotherapy1 and increase the therapy’s efficacy in clinical settings.
What are TSA-based mIHC assays?
Under the umbrella of mIHC, various methods can be used to obtain multiplexed data. Tyramide Signal Amplification (TSA) is an mIHC technique designed to increase the detection of low-abundance targets. TSA can be integrated into conventional immunohistochemistry (IHC) protocols to achieve multiplexed proteomic data or to improve assay precision and sensitivity2.
Unlike conventional chromogenic IHC, where Horse Radish Peroxidase (HRP) converts the diaminobenzidine (DAB) substrate into a brown precipitate in the vicinity of the protein of interest, TSA-based IHC follows a different approach to produce a fluorescent signal. In this case, the HRP will convert a fluorescently-labeled tyramide substrate into a reactive form that covalently binds to tyrosine residues on and near the protein of interest3.
Thanks to the covalent bonds created, the target proteins in a TSA assay are stained sequentially, washing away the antibodies between cycles while keeping the labeled tyramide in place, and all markers can be detected simultaneously at the end of the assay. For each marker, the process is as follows: firstly, the primary antibody and the HRP-conjugated secondary antibody are incubated. Next, the TSA substrate is incubated and covalently binds in the neighborhoods of the protein of interest. Finally, the antibodies are eluted, leaving only the bound TSA reagent behind. The process can be repeated up to seven more times before the tissue is imaged with a fluorescent microscope or a spectral microscope for higher plex assays.
What makes TSA-based mIHC assays challenging?
TSA-based assays can cause researchers many frustrations:
- the high number of manual steps that need to be carefully performed;
- the long protocol duration times;
- the high consumption of TSA reagents.
These challenges are compounded by the complex optimization process requiring multiple cycles before an assay is ready to be used.
How can automation be used to tackle the challenges of TSA assays?
Automation can be integrated into workflows to tackle the main challenges of TSA-based assays. It reduces the amount of time spent at the bench and the quality and reproducibility of the data generated.